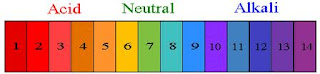
- Using figure 1.44 on page 70, decide which is more acidic:
- A soft drink or a tomato- A soft drink is more acidic with a pH of 3
- Black coffee or pure water- Black coffee is more acidic with a pH of 5
- Milk of magnesia or household ammonia- though both are considered basic milk of magnesia is more acidic with a pH of 10.
- A solution at 2.0 pH is 100 times more acidic then a pH of 4.0
- Three negative affects of inappropriate pH levels on aquatic organisms: low pH (highly acidic) impair fish egg development, also low pH increases the concentration of metal ions in natural waters that are toxic to fish in high concentration, and alkaline solutions are harmful because they are able to dissolve organic materials like skin and scales.
- Polar molecules have an uneven distribution of electrical charges usually with a negative charge on one end and a positive charge on the other and dissolve in polar solvents; non-polar molecules only dissolve in non-polar solvents.
- I would select lamp oil and ethanol to dissolve and non-polar molecule because non-polar molecules dissolve in non-polar solvents such as oil.
- Table salt dissolves in water because it is a polar solute and it does not dissolve in oil because oil is a non-polar solvent.
- The phrase “like dissolves like” refers to the patter in which polar molecules dissolve in polar solvents only and non-polar solutes dissolve in non-polar solvents only.
- A person cannot satisfactorily clean greasy dishes with just plain water because greasy is a non-polar solute and water is a polar solvent; therefore, the grease cannot dissolve or be cleaned with just plain water.
Monday, June 27, 2011
Homework #1 week three
Subscribe to:
Post Comments (Atom)

No comments:
Post a Comment